Introduction: ZUMA-7 is the largest randomized controlled trial (RCT) for chimeric antigen receptor (CAR) T-cell therapy which compared axicabtagene ciloleucel (Axi-cel) to standard of care (SoC) including autologous stem cell transplant (ASCT) for the treatment of relapsed or refractory DLBCL. Both arms of ZUMA-7 contained potentially curative treatments; however, differences in the treatment completion rate (94% received axi-cel and 36% received ASCT) and timing to receive definitive treatment led to differences in the extent and timing of cure. Furthermore, 57% of the SoC arm received potentially curative CAR-T therapies in subsequent lines of therapy leading to a delayed cure in that fraction of the population. The resulting event-free survival (EFS) and overall survival (OS) curves can pose challenges in interpretation and extrapolation for the purpose of simulations of long-term treatment effects and cost-effectiveness. Mixture cure modeling (MCM) has been suggested as a superior method to evaluate treatments of a curative nature to allow for part of the population to achieve long-term remission. This method has been shown to be useful both in designing and powering clinical studies of curative therapies but also for extrapolation of long-term outcomes in simulation studies. However previous iterations of this method did not account for differences in timing of curative therapies. ZUMA-7 had two pivotal analyses separated by nearly 2-years of follow-up: a primary EFS (datacut 1) and a primary OS analyses (data cut 2). The aim of this study was to use the two data cuts of ZUMA-7 to validate MCM in 2L LBCL and describe differences that may have arisen due to treatment specific timing and extent of cure.
Methods: Overall survival individual patient-level data from the ZUMA-7 trial for axi-cel and SoC from both data cut 1 (median follow-up: 24.9 months) and data cut 2 (median follow-up: 47.2 months) were used in this analysis. Three different parametric modeling approaches were applied and compared in both data cuts: standard parametric model, spline-based model, and MCMs. Modeling approaches were evaluated for plausibility across a range of metrics, across the two data cuts, including visual fit, plausibility of long-term estimates, statistical goodness of fit, inspection of hazard plots, point-estimate accuracy, and conditional survival estimates. Additionality, the ability of the early data cut extrapolation to predict the actual data was assessed by comparing the model predictions with the latest KM estimator in both trial arms. Differences between trial arms were evaluated.
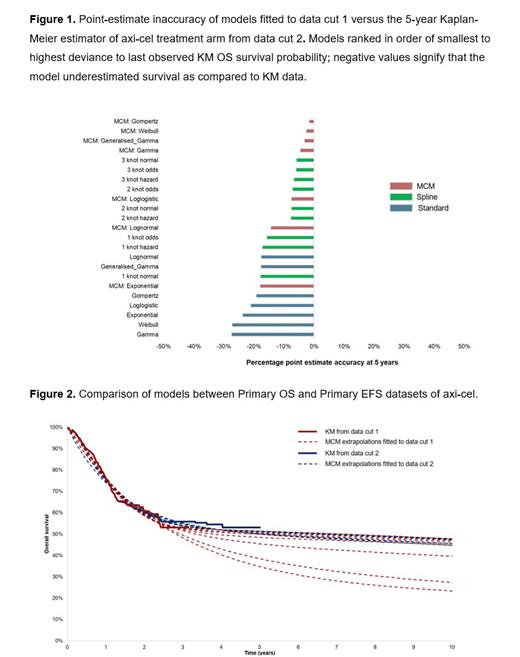
Results: Cure-based and spline models provided the best fit based on the percentage inaccuracy of extrapolations fitted with data cut 1 in comparison to 5-year OS Kaplan-Meier estimates confirmed by data cut 2. In the axi-cel treatment arm, the percentage inaccuracy range between 2% and 27% (Figure 1). Based on data cut 1, cure fractions ranged considerably across the MCM extrapolation forms considered for both treatment arms (range: 24-54% [Figure 2] and 35-49% for axi-cel and SoC, respectively). Notably, the range of cure fractions narrowed considerably based on extrapolations performed on the more mature data cut 2 (range: 50-54% [Figure 2] and 41-50% for axi-cel and SoC, respectively). Overall, the uncertainty in the estimate of cure fractions based on MCMs was reduced from data cut 1 to data cut 2 for axi-cel vs. SoC, suggesting clinical input is needed to validate early extrapolations using less mature data cuts.
Conclusion: Our analysis suggests that MCMs are helpful in making accurate predictions of survival based on immature data when a plateau in the survival curve is clinically plausible. However, this analysis also highlights the importance of considering clinical plausibility to guide specific curve selection in the presence of immature survival data, as there was a high degree of variability in the early extrapolations which was reduced with the longer-term follow-up. The ability to reliably extrapolate from early data may improve clinical decision making and reduce delays in patient access to potentially lifesaving treatments as more mature follow-up data are being collected.
Disclosures
Patel:Kite, A Gilead Company: Current Employment, Current holder of stock options in a privately-held company, Other: Current holder of stock in a privately-held company. Ray:Kite, a Gilead Company: Current Employment. Rodriguez-Guadarrama:Maple Health Group,LLC: Current Employment. Smith:Maple Health Group,LLC: Current Employment. Neumann:Kite, a Gilead Company: Current Employment. Blisset:Maple Health Group,LLC: Current Employment. Xue:Biogen: Current holder of stock options in a privately-held company, Ended employment in the past 24 months; Gilead Sciences: Current Employment, Current holder of stock options in a privately-held company; Kite, a Gilead Company: Other: Travel Support.